In life sciences, innovation and regulation are always in balance. Organizations must carefully evaluate the impact of every change on compliance, which is why digital transformation and data adoption have historically been measured and deliberate. This cautious approach ensures that medicines remain safe, effective, and aligned with regulatory expectations.
When I worked at CSL Behring, cloud technology was still viewed with skepticism, especially by IT infrastructure teams. Their caution reflected a larger truth: in a highly regulated industry, every new technology or process must be vetted to protect product integrity and patient safety.
Change and Measurement
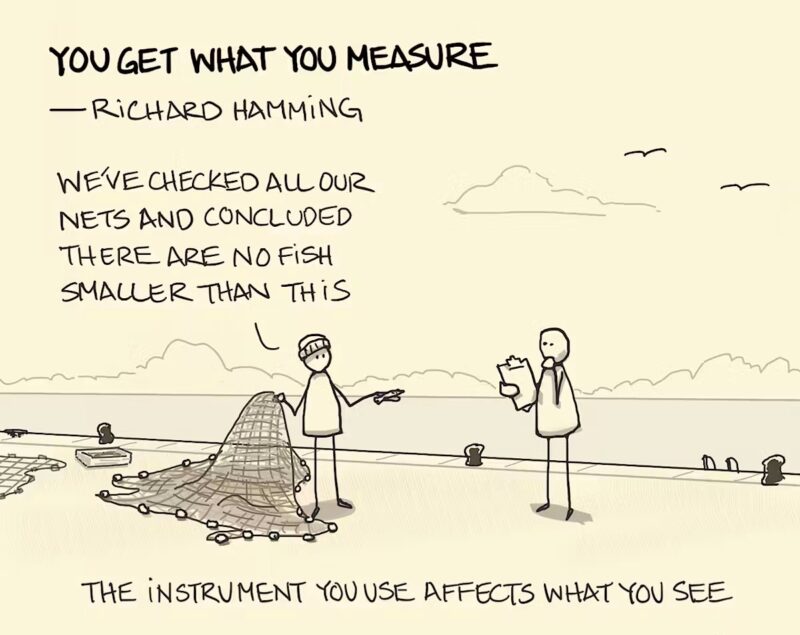
Change is hard in all disciplines, and data science is no different. Bias is inherent in any analytical process, and data scientists go to great lengths to eliminate bias and to be as “objective” as possible. Richard Hamming (1915-1998) is credited with coining the phrase “You get what you measure.” Hamming was the inventor of Hamming Codes, a technique for correcting errors in data transmission and computer memory, which is still in use today.[1]
The effectiveness of a manufacturing process is measured by its ability to consistently produce medicines that deliver on the promise of clinical trials. ISO standards require manufacturers to demonstrate ongoing quality assurance, not just one-time checks, which makes laboratories indispensable to the process. Losing the ability to test and measure means production must stop — a costly event in any pharmaceutical operation.
As a result, life science organizations tend to be very conservative when it comes to change, carefully evaluating the impact of any change on compliance. When I joined CSL, there was cloud, and cloud delivery was viewed with suspicion, especially by the infrastructure team, which also saw this as a threat to their jobs. In fairness, however, my colleagues were risk-averse, and their caution also reflected a larger truth: in a highly regulated industry, every new technology or process must be vetted to protect product integrity and patient safety.
Quality
This brings us to Quality – one of the most critical areas for life science businesses.
All life science professionals are keenly aware of the role testing laboratories play in the manufacture of pharmaceuticals. I was new to the term Quality when I started at CSL, however, I quickly learned that the term has a particular and essential meaning for a life science company.
The effectiveness of the manufacturing process is measured by its ability to consistently produce medicines that deliver on the promise made in the clinical trial process: that the medicine will be effective in treating the patient’s illness. There are many international standards that all regulatory agencies leverage to define the benchmarks life science companies must meet to prove that their products and services consistently meet customer and applicable statutory and regulatory requirements. These Regulatory agencies require manufacturers to demonstrate ongoing quality assurance, not just one-time checks, which makes laboratories indispensable to the process.
These standards require companies to:
- Facilitate opportunities to enhance customer satisfaction.
- Address risks and opportunities associated with its context and objectives.
- Demonstrate conformity to specified quality management system requirements.
Manufacturing QA Labs
This brings me to the necessity of Quality Assurance (QA) Labs in the manufacturing process. Losing the ability to test and measure the manufacturing process means production must stop. Obviously, stopping a manufacturing facility, or at least the production lines the lab serves, is necessary.
The laboratory process is continuous and highly regulated throughout the manufacturing process (pharmaceuticals, biologics, medical devices). The frequency depends on the type of product, the stage of manufacturing, and regulatory requirements. Manufacturers must demonstrate ongoing quality assurance — not just one-time checks.
Here is a breakdown of the types of tests.
- Raw Materials and Incoming Goods – Every batch of raw materials (active pharmaceutical ingredients, excipients, solvents, packaging) is sampled and tested before use. Some companies operate on reduced testing programs if vendors are certified and have strong quality agreements, but release testing is still required.
- In-Process Controls (IPC) – Measurements are done during manufacturing, often at critical steps (e.g., blending, granulation, fermentation, purification). IPC ensures the process stays within validated parameters, preventing deviations before the final product stage.
- Finished Product Testing – Every batch of medicine must undergo a complete set of quality control (QC) tests before release. This includes potency, purity, sterility (if applicable), dissolution, stability, and other product-specific criteria. A batch cannot be released until it passes all required QC tests.
- Environmental & Facility Monitoring – Cleanrooms and production environments are monitored continuously or daily, checking air, surfaces, and personnel for contamination.
- Stability Testing – life science companies conduct stability testing periodically over the product’s shelf life (e.g., 0, 3, 6, 12, 24 months) to ensure medicines remain safe and effective until expiry.
- Regulatory Oversight – The life sciences industry is one of the most regulated in the world, for obvious reasons. Good Manufacturing Practices, set by international standards agencies, dictate the frequency and rigor of testing.
- Good Manufacturing Practice (GMP) Standards – Standards set by agencies like the Food and Drug Administration FDA (U.S.) [2] [3], the European Medicines Agency (EMA) (Europe)[4], and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines[5].
This testing regime is the backbone of how life science companies operate their manufacturing laboratories, ensuring that every batch of medicine undergoes rigorous, documented testing before it reaches patients.
This information also has significant value not just to patients and physicians but also to the life science companies themselves. These companies use this information to improve the manufacturing process, thereby enhancing efficiency, increasing yields from raw materials, and reducing the costs involved in the manufacturing process.
This represents terabytes of data that data teams can use to lower costs and increase profit margins by reducing waste, improving efficiency, and enhancing the overall product quality. At CSL, I participated in several leadership conversations exploring data protection and why this was essential for business operations. The justification for enhancing data protection was the value of this information. Manufacturing leaders explained that improving manufacturing yield by just 1% to 2% would substantially increase profitability. Analyzing lab data could allow us to achieve this result.
As I pointed out in the first part of this series, life science companies are at the top of the list of industries that feature intangible assets on the plus side of their balance sheets – with good reason!
Green Leaf’s data team excels at uncovering value in data and demonstrating how it can enhance business results. Green Leaf focuses on transforming your resources into a data–driven decision-making powerhouse by increasing your data output, giving you more time for business insights and smarter decisions.
In summary, Hamming is right! You do get what you measure. Manufacturers must demonstrate ongoing quality assurance, not just one-time checks, and data powers this process.
Coming up next in this series, we’ll begin to look at new technology that is revolutionizing not only Data Analytics in the life science space, but in other industries as well. Thanks for reading!
References
- Hamming,Hamming, “You Get What You Measure” (June 1, 1995). 1995: YouTube.
- Adminstration, U.S.F.a.D.Current Good Manufacturing Practice (CGMP) Regulations2025 [cited 2025].
- FDA), U.S.F.a.D.A.U.,21 CFR Part 314, U.S.F.a.D.A.U. FDA), Editor. 2025, United States Food and Drug Agency (US FDA): Washington, DC.
- (EMA), E.M.A.European Medicines Agency (EMA). 2025 [cited 2025 September 2]; The European Medicines Agency (EMA) is a decentralized agency of the European Union (EU). It is responsible for the scientific evaluation, supervision, and safety monitoring of medicines.]. Available from: European Medicines Agency (EMA).
- (ICH), T.I.C.f.H.ICH Quality Guidelines. 2025 [cited 2025 September 2]; Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.